The team believes that their breakthrough can provide a safer and more environmentally friendly substitute for lithium-ion batteries which currently dominate the battery market. There are currently some challenges in the use of lithium-ion batteries in solar energy. They charge slowly. If they are punctured, damaged or the environment is not ideal, the active lithium-ion may catch fire. In the current research, scientists are using aluminum and zinc to make a new cell.
The researchers said that an interesting feature of their batteries was that it had only two elements for the anode and cathode. These elements were aluminum and carbon. These two elements were inexpensive and environmentally friendly. The life cycle of the battery is very long. When the team calculated the cost of energy storage, the longer the battery's service life is, the more obvious the cost of energy storage will be reduced.
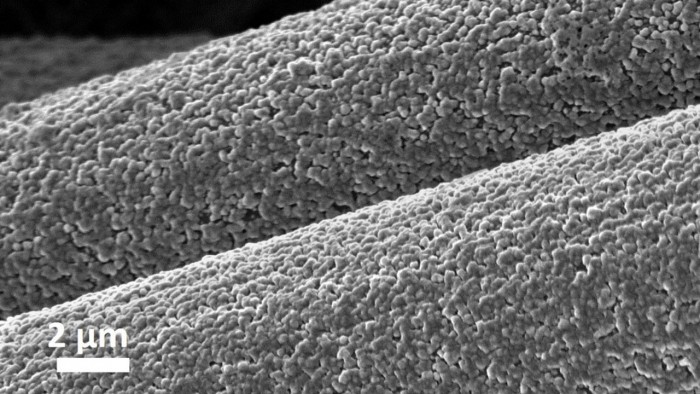
One of the main advantages of using aluminum is that aluminum is a rich material with high energy storage capacity, compared with many other metals. The challenge is that aluminum is difficult to integrate into battery electrodes. Aluminum will chemically react with the glass fiber separator that physically separates the anode and cathode, causing the battery to short-circuit and fail.
Researchers at Cornell University solved this problem by designing a matrix made of interwoven carbon fibers to form better chemical bonds with aluminum. When the battery is charged, aluminum is deposited into the carbon structure through covalent bonds. This technology requires a non-planar structure of the battery, and a deeper and more consistent aluminum layering should be created, which can be finely controlled. The researchers pointed out that under actual conditions, their aluminum anode batteries can be reversibly charged and discharged one or more orders of magnitude more than other aluminum rechargeable batteries.